Thursday 10th July 2025
The Chemistry of Paracetamol by Saskia Haley
Press Enter to search or ESC to close
Thursday 10th July 2025

TLDR: Although paracetamol, the main ingredient in painkillers, is widely used, few appreciate its fascinating chemistry. Also called acetaminophen, it’s an organic molecule made up of a hydroxyl group (-OH), a benzene ring, and a secondary amide (HNC=O). It was first synthesised by Harmon Northrop Morse, though he was unaware of its medicinal properties, which were only discovered when acetaminophen was found in urine of patients who had taken other painkillers like phenacetin. Its exact mechanism of action is yet to be determined, but we do know that it inhibits COX-1 and COX-2 pathways, which are responsible for the production of prostaglandins, molecules which cause our feelings of pain, and its action on the hypothalamus is what causes its fever-reducing properties. When paracetamol is broken down in the liver, it can form a reactive metabolite which can cause liver failure, but this is only an issue when an overdose is taken, and the concentration in the blood is highest 90 minutes after it’s taken orally. Paracetamol is manufactured by first synthesising nitrobenzene via electrophilic substitution. Further reaction with zinc and ammonium chloride, and then dilute acid (and finally acylation), produces the paracetamol molecule. The main potential side effects are cardiac issues, especially for those over 65, but it doesn’t tend to be a problem when taken at the correct dose. However, recent research has suggested that taking paracetamol increases risk-taking behaviour. Random facts: it’s toxic for brown tree snakes and is also used in hair dye to stabilise hydrogen peroxide!
-----------------------------------------------------
Imagine waking up in the morning, groggily forcing your eyes open, groping around to shut off your alarm, only to find your nose is completely blocked. Snuffling, you swallow down some water, and find your throat is dry and sore, like daggers are cutting into your oesophagus. Wincing in pain, you try to stumble out of bed, your bones aching with the effort, and your head pounding like your brain is trying to break out of your skull. You pull out a tissue and sneeze self-pityingly, resigned to the only obvious fact your mind seems capable of processing: you're not well.
While in the 19th century, cold-and-flu-like symptoms may have been cause for concern, nowadays, most of us would just take a couple of paracetamol tablets and soldier on. We appear to just swallow these pills without a second thought now, having learned that they can magically make any pain we feel disappear, be it earache, a raised temperature, or a sore throat. But what is actually inside this 'magic' medicine, and how does it work?
What is paracetamol?
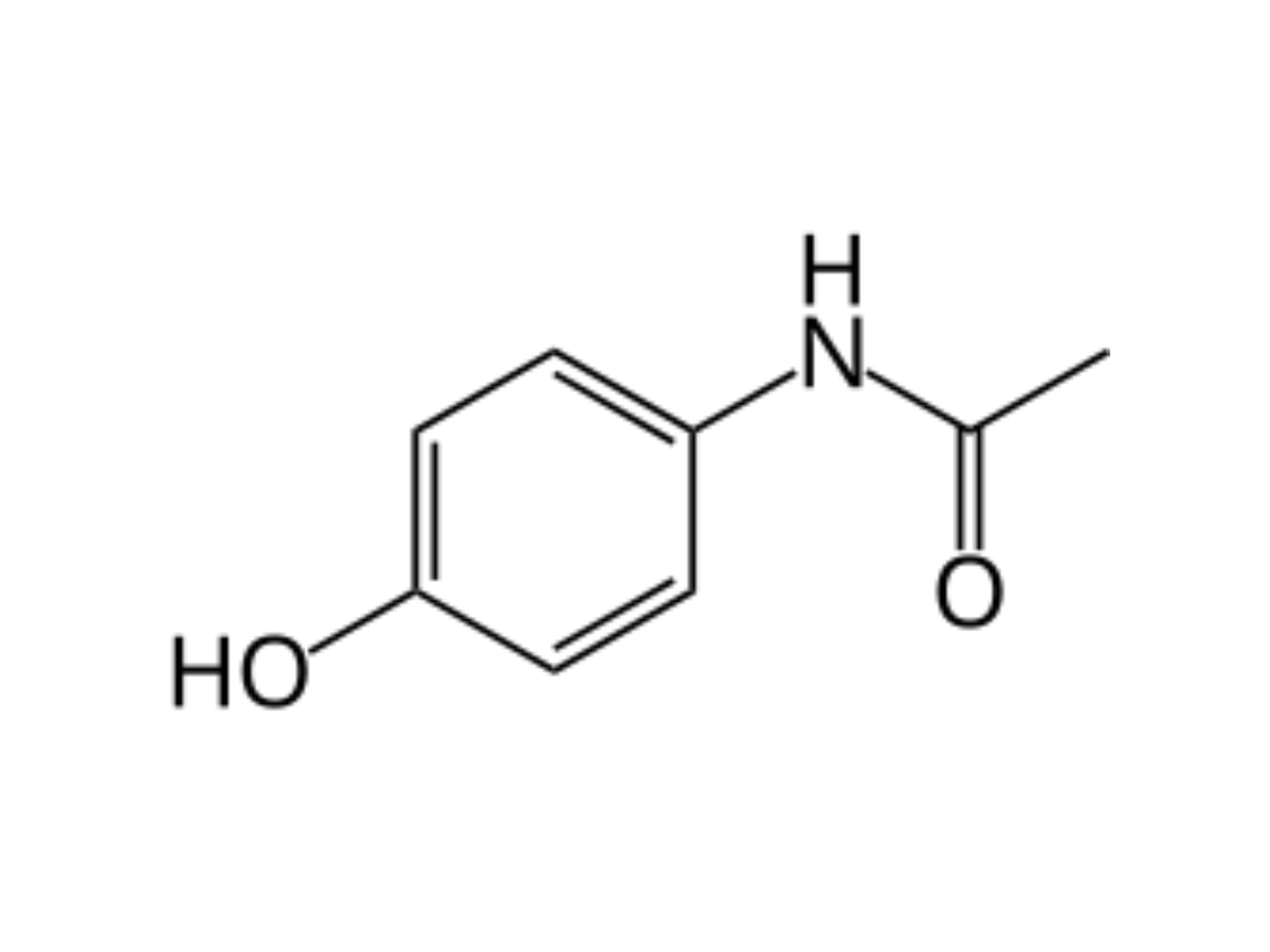
Paracetamol, or acetaminophen, is an organic molecule which is the main ingredient in modern painkillers. In its pure state, it is a white crystalline powder (it melts at 170oC), and although odourless, it has a distinct bitter taste (which is why trying to chew the tablets instead of swallowing them really isn’t pleasant). It is relatively stable, but reacts with aspirin, which is why you should leave a 4-hour gap if taking both, to reduce the possibility of side effects. The formula pictured in Fig. 1 (see bottom of page) is the skeletal formula, meaning carbon atoms are omitted and all spare bonds are assumed to be filled with hydrogens. I have highlighted the three functional groups. For anyone wondering, the IUPAC name is N-(4-hydroxyphenyl)ethanamide, the molecular formula is C₈H₉NO₂, and the relative molecular mass (Mr) is 151.165.
How was paracetamol discovered?
Before the invention of paracetamol, a variety of methods were used to treat symptoms of illness. Scientists found that certain chemicals found in white willow and cinchona tree bark, known as salicins, had antipyretic (fever-reducing) effects in the body, and this was the basis for modern-day aspirin. However, these trees became scarce in the 1880s, leading scientists to consider two alternatives: acetanilide (this is almost the same molecule as paracetamol, just without the -OH group) and phenacetin (which is also similar to paracetamol, and has the -OH substituted for CH3CH2O).
Around this time, Harmon Northrop Morse had already synthesised paracetamol, but was unaware of its potential medicinal properties. It was only in 1893, when scientists found the molecule present in the urine of patients who had taken phenacetin, that its usefulness was suggested. This was further supported in 1948, when it was found that the analgesic (pain-relieving) nature of acetanilide was caused by how it reacts with chemicals in the body to form paracetamol, which is what was actually responsible for the drug’s effects.
Because acetanilide was linked with a reduction in the capacity of the blood to transport oxygen to cells, and phenacetin was even linked with cancer, both were deemed unsafe, so the use of paracetamol itself (which was harmless in comparison) was advocated instead. It first went on sale in the US, under the brand name Tylenol, in 1955. Finally, in 1956, 500mg tablets were introduced in the UK under the name Panadol, and it was quickly bought in preference to aspirin as it did not irritate the stomach.
Structure of a paracetamol molecule and its functional groups
Functional groups are atoms (or more commonly groups of atoms) which give a homologous series (a family of similar molecules where each member differs from the previous by CH2) their characteristic chemical properties. For example, alkenes all have a C=C double bond, which allows them to undergo addition reactions. In paracetamol, the three functional groups are a benzene ring, a hydroxyl group, and a secondary amide group. But what are these groups, and how do they affect paracetamol’s mechanism of action?
Benzene
Compounds containing benzene rings are known as aromatic, due to benzene’s strong sweet smell. One example which you’ve probably come across without realising is thymol, the chemical responsible (unsurprisingly) for the aroma of the herb thyme, which is a benzene ring with an -OH group and several alkyl branches (see Fig. 2). Unfortunately, despite its sweet scent, benzene is not a completely innocent molecule. Found in cigarette smoke, it has been labelled a carcinogen (a substance which may cause cancer).
Michael Faraday, an English scientist, first isolated benzene in 1825, after realising it was the oily residue from gases used in street lighting. Although, despite Faraday working out its molecular formula, the structure of the compound remained a mystery. August Kekulé, a German scientist, suggested the benzene structure could have alternating single and double bonds (this model of benzene is shown in the cover picture of this article), and was supposedly inspired by a dream of a snake biting its own tail. However, this model did not gain the support of all chemists, as benzene did not appear to exhibit the expected chemical properties for a cyclic alkene. For example, from GCSE Chemistry, we know that alkenes decolourise orange bromine water, as the double bond can break to form a dibromoalkane (via a mechanism called electrophilic addition). But benzene did not have this effect on aqueous bromine, causing scientists to question whether there were really any C=C bonds present. Further arguments arose when Kathleen Lonsdale, an Irish crystallographer, examined benzene by X-ray diffraction in 1929, finding that all the carbon-carbon bonds were exactly the same length, at 0.139nm. Even more surprisingly, this length was between the value expected for a single bond (0.153nm) and a double bond (0.134nm), hinting that the bonds present were somehow a cross between the two. The enthalpy of hydrogenation also didn’t fit the expected structure.
Therefore, in light of all the evidence, scientists were forced to develop a new model of benzene, and so the ‘delocalised’ structure we recognise today was born. This model shows a system of pi bonds (a type of covalent bond) above and below the carbon ring caused by overlapping p-orbitals (components of electron shells) containing the unbonded electron from each of the six carbon atoms. It is like each carbon atom has one and a half bonds with other carbon atoms. The delocalised model of benzene is denoted by a hexagon with a circle inside it (see Fig. 3).
Phenol
The -OH group and the benzene ring it is attached to can be referred to together as phenol.
Although the -OH group is the functional group of the homologous series of alcohols (such as ethanol, with two carbon atoms, which is what we actually mean when we refer to alcohol in daily life), phenol undergoes quite different reactions to the alcohols, due to the -OH being directly bonded to the aromatic ring.
Much like in alcohols, the oxygen atom of the O-H bond is more electronegative (pulls the electrons in the covalent bond further towards it) than the hydrogen, meaning the bond has a charge difference across it, with the O being slightly negative and the H being slightly positive; we call this a polar bond. Due to the polarity of the bond, hydrogen bonds (attraction between the slightly positive hydrogens and slightly negative oxygens on different molecules) can form between phenol and water molecules (H2O has two O-H bonds), meaning phenol is soluble. Although, it is less soluble in water than the alcohols, as the non-polar benzene ring cannot dissolve (as it can’t attract the water molecules as there are no partial charges). When phenol is dissolved, however, the oxygen atom completely takes the two electrons in the O-H bond, causing the H atom to leave the molecule as an H+ ion (which is just a proton), and forming a phenoxide ion (see Fig. 4). Since it releases protons when dissolved in water, we can call phenol a weak acid (weak because only some of the molecules dissociate (break apart) in this way).
Phenol reacts via electrophilic substitution more readily than benzene and does decolourise bromine water. Three Br atoms can swap for H atoms on the aromatic ring, forming a white precipitate of 2,4,6-tribromophenol and hydrogen bromide. (If the bromine atoms on the 2,4,6-tribromophenol were chlorine atoms instead, you’d get 2,4,6-trichlorophenol, which is what we call the antiseptic TCP.) The reason for this difference in reactivity between phenol and benzene is that phenol has an oxygen atom. As we’ve established, oxygen is very electronegative, and has two lone pairs of electrons on its atom as well. This increases the density of negative charge in the molecule, so phenol attracts electrophiles like Br2 and NO2+ (electron pair acceptors – i.e., substances which want to gain more electrons) more readily. In the case of NO2+, it wants to gain electrons as it is positively charged, but when the Br2 molecule approaches phenol, phenol has a sufficiently high electron density that the Br-Br bond becomes polar, with the closest Br to the phenol developing a partially positive charge, so it is then attracted towards the aromatic ring.
Secondary amide
Amide groups contain a carbon double-bonded to an oxygen and single-bonded to a nitrogen. The nitrogen can have any number of hydrogens and alkyl groups attached to it, as long as it makes no more than three bonds in total. Amides are commonly found in nature, as they are essential functional groups in proteins, and are formed in the reaction between acyl chlorides and amines or ammonia (depending on whether the amide is primary, secondary or tertiary).
In paracetamol, a secondary amide is present, since the nitrogen is bonded to two carbons (and one hydrogen). Since amides have the highest priority in nomenclature out of all the groups found in paracetamol, the other two groups become prefixes in the name (benzene goes to phenyl, alcohol goes to hydroxy), while -amide is used as a suffix. The N- in the N-(4-hydroxyphenyl)ethanamide is used to signify that the hydroxyphenyl is attached to the nitrogen atom. This is similar to how we use numbers to name branched alkanes to show which carbon atom the branches are attached to (e.g. 2-methylpentane shows there is a -CH3 group on the second carbon atom in the molecule).
Amides are stronger acids than amines, since the lone pair of electrons on the nitrogen atom can move to become a double bond between the nitrogen and carbon atoms, forcing the C=O bond to break and become a single bond (as carbon can only ever have 4 bonds) – this pair of electrons which was the bond then becomes a lone pair on the oxygen atom. Since the nitrogen atom has given up electrons, and the oxygen atom has gained them, the nitrogen has a 1+ charge and the oxygen a 1- charge (this makes sense as the overall molecule must be neutral as it was neutral to start with – see Fig. 5). Therefore, in reality, the structure is somewhere between having a double bond between the C and O, and a double bond between the C and N, so it forms a sort of halfway arrangement (a bit like in benzene when each carbon had 1.5 bonds). This is called a resonance structure, and can be drawn with a dashed line (see Fig. 6). Since the nitrogen atom has a sort of half positive charge, it is electron deficient, meaning it is less able to attract the H atom it is bonded to, so an H+ ion can be lost relatively easily from the molecule, making it acidic.
Amides undergo many interesting organic reactions. In water, they can undergo hydrolysis (splitting up in solution), to form a carboxylic acid (such as ethanoic acid, which is the main ingredient of vinegar) and either ammonia or a primary amine, depending on whether it is a primary or secondary amide. They can also be dehydrated with P2O5 to form a nitrile (C triple-bonded to N), which can be very useful for further synthesis reactions of other organic compounds.
How does paracetamol work?
Although the exact mechanism of action of paracetamol is still yet to be fully discovered, scientists do know that the ability of the -OH group to hydrogen bond enables it to inhibit cyclooxygenase (COX) pathways. Two of these enzymes, COX-1 and COX-2, are involved in the synthesis of prostaglandins (chemicals responsible for pain sensations, among other things), so by preventing their function, paracetamol reduces the concentration of prostaglandins in the body, and so the pain felt by the patient. However, the production of COX is only inhibited in central tissues, so the medication does not have any undesirable effects elsewhere. Interestingly, while aspirin reduces pain by permanently rendering the COX enzymes unusable by fully blocking their active sites, paracetamol is thought to block the enzymes indirectly and reversibly, so it is more temporary.
On the other hand, paracetamol which is administered straight into the bloodstream (rather than orally) can be broken down not only in the liver, but also in the walls of blood vessels. This creates a chemical by-product which makes blood vessels relax, resulting in a (sometimes fatal) drop in blood pressure (but this is not an issue when taken orally and at the standard recommended dose). However, the main takeaway from this is that paracetamol injected into the bloodstream may have its pain-relieving properties caused by a metabolite which is not produced in the liver but locally all over the body in the arteries. Further research is currently being carried out on this, and whether the same is true for oral paracetamol.
Its antipyretic (fever-reducing) effects are believed to result from direct action on the hypothalamus, a part of the brain which plays a role in regulating temperature (among other functions). Stimulating certain receptors in the hypothalamus results in vasodilation (widening) of blood vessels near the surface of the skin, as well as increased sweating, causing body heat to be lost, reducing a high temperature.
Paracetamol is mainly metabolised (broken down) in the liver, where it can form a reactive product called N-acetyl-p-benzoquinone imine (NAPQI) which binds to liver cells, reacting with essential molecules inside them like lipids and proteins. It can therefore cause liver failure in high doses, and this is exacerbated further by excessive alcohol consumption. In safe doses, however, NAPQI undergoes further reaction very quickly with glutathione (a substance made from certain amino acids which is found in many fruits and vegetables like asparagus and avocado), forming harmless amino acids. After 8 hours, almost all paracetamol taken is metabolised, and only traces are present (the concentration in the blood plasma is highest 90 minutes after it is taken orally).
How is paracetamol manufactured?
In order to synthesise paracetamol, nitrobenzene must first be produced via an electrophilic substitution reaction. This involves the reaction of benzene with nitric acid, in the presence of a concentrated sulfuric acid catalyst and a temperature of about 55oC.
The first step of this is the reaction of the sulfuric and nitric acids:
HNO₃ + H₂SO₄ --> HSO₄⁻ + NO₂⁺ + H₂O
Next, the nitronium (NO₂⁺) ions generated react with benzene via electrophilic substitution. The positive nitronium ion is attracted towards the benzene ring (which has a dense negative charge due to the delocalised electron structure), and two of the electrons from the benzene ring are donated to form a bond between the nitrogen atom and a carbon on the benzene, which also has a hydrogen attached to it. This means that the molecule now has a 1+ charge, so this hydrogen atom is lost as a proton, leaving nitrobenzene (see Fig. 7).
It is called electrophilic substitution because the nitronium ion is an electrophile, and it substitutes one of the hydrogen atoms on the benzene molecule. The proton released then reacts with the hydrogen sulfate ion produced earlier on in the first step, regenerating the sulfuric acid catalyst (although it technically takes part in the reaction, it is not used up overall, so can be called a catalyst). This keeps the reaction going.
Once nitrobenzene has been produced in this way, it is reduced with zinc and ammonium chloride under a high temperature, forming N-phenylhydroxylamine. This is then reacted with a dilute acid (usually HCl or H2SO4), forming aminophenol. Since the aminophenol formed has the -NH2 and -OH groups on opposite carbon atoms, the prefix para- is used (this denotes groups on positions 1 and 4 in the molecule), so this product is called para-aminophenol. The final step involves acylation (adding a -C=O group into the molecule), forming paracetamol (see Fig. 8). This is why we call it paracetamol, as the attached groups are on opposite carbons of the benzene ring.
Are there any negative effects of paracetamol?
A disconcerting study conducted in 2022 revealed that taking one paracetamol tablet per day can raise the risk of a fatal heart attack or stroke by 20%. Therefore, it is important to take paracetamol only when you really need it – this also reduces expenses. Long-term use also increases risk of heart disease in those with high blood pressure, and has been shown to increase tiredness and breathlessness in some patients. According to the US Food and Drug Administration (FDA), it has even been associated with a risk of rare but serious skin conditions, which can in some cases be fatal.
In terms of the age groups most vulnerable to side effects, research has suggested that paracetamol is less safe for those over 65 than previously thought, as it was associated with an increased risk in gastrointestinal, kidney and cardiac problems.
Another concern is that certain formulations of the medicine have a high salt content, which aggravates heart problems; some have even been shown to contain 1.5 times the daily sodium intake for adults, just in one adult dosage. This is equivalent to eating over three McDonald’s Big Macs! After a study conducted in the UK, patients on this version of the tablets were also 45% more likely to suffer a heart attack, heart failure or a stroke within a year than those on the low-sodium version.
On the other hand, overdoses of paracetamol can be even more severe. It can lead to liver and kidney damage (due to the formation of NAPQI which I mentioned before), and this can be lethal. However, as long as you take the correct dose, for no longer than about three days at a time, it is very rare to experience any side effects. Even with prolonged use, patients do not develop tolerance or physical dependence, though some may feel psychologically dependent on it.
But, scientists are becoming increasingly sure that paracetamol has a strange effect on the functioning of certain parts of the brain. It was shown to increase risk-taking behaviour and reduce anxiety associated with dangerous situations after 545 adults participated in a balloon test. Those under the influence of paracetamol were more likely in all cases to let the balloon grow larger before stopping, despite the increased risk of it popping, than those taking a placebo pill. (The graphs can be seen on PubMed – link below.)
Another danger of paracetamol is that when it is heated to decomposition (though I’m sure nobody would try this with their painkiller tablets!), toxic carbon monoxide (CO) gas is released. This binds with haemoglobin on red blood cells permanently, rendering the cells useless as they can no longer take up oxygen, reducing the capacity of the blood to transport O2 to cells for respiration. To make matters worse, CO is colourless and odourless, so it is impossible for the victim to notice they are being poisoned.
However, just count yourself lucky that you’re not a brown tree snake. Paracetamol is lethal to these animals, and for this reason, it is used to control their populations in some parts of the world. It is also harmful to aquatic life.
The alternatives available are mainly Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), like Ibuprofen or aspirin. Ibuprofen tends to be better for pains concentrated in one area, like toothache and sprains, while paracetamol can work more effectively for headaches and stomach aches where the location of the pain is more general. Paracetamol, unlike some NSAIDs, is the most popular painkiller because it only rarely causes an allergic reaction, so it is suitable for people with asthma.
Incidentally, paracetamol is even used in hair products, as it stabilises dyes like hydrogen peroxide. But that doesn’t mean you should drink hair dye when you have a sore throat!
To conclude…
Paracetamol is a molecule we have all come to rely on in our daily lives, but few appreciate its fascinating chemistry. I therefore hope that this article has been interesting to you in understanding its hidden secrets. If you want to know more about it, I’d recommend these five links, which I found highly useful when writing this. Thanks for reading!
1. Side effects: Experts issue warning after world's most common painkiller has a bizarre and unexpected side effect | Daily Mail Online (or Effects of acetaminophen on risk taking - PubMed for a more scientifically accurate summary)
2. This document from RSC 2002 has just about everything, and even some questions and tasks if you feel so inclined!: https://edu.rsc.org/download?ac=11243
3. OCR textbook p.438 if you are studying Chemistry A-level
4. Information on amides: Amides | Encyclopedia.com
5. Detailed information on properties: PubChem: Acetaminophen